FGI: OH → F / DAST - 01
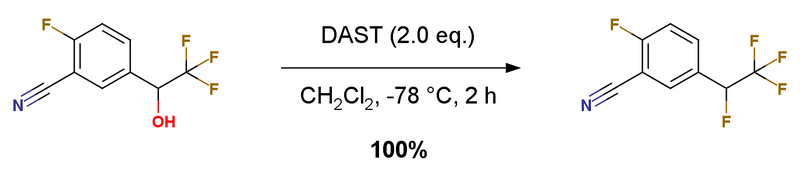
2-Fluoro-5-(1,2,2,2-tetrafluoro-ethyl)-benzonitrile 2-Fluoro-5-(2,2,2-trifluoro-1-hydroxy-ethyl)-benzonitrile (1 g, 4.56 mmol) in DCM (5 mL) was treated with DAST (Aldrich, 1.12 mL, 9.13 mmol) at -78 ℃ for 2 hours. The reaction was quenched with saturated NaHCO3 and extracted with DCM. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated to give the crude material, purified by silica gel column (hexanes: ethyl acetate 4:1) to afford the title compound. Colorless solid, yield 1.01 g, 100%.
Intermediate 35 (1 g, 3.1 mmol) was suspended in DCM (7.7 mL) and the reaction was cooled down to 0 °C. Then DAST (0.45 mL, 3.7 mmol) was added dropwise. After 20 min at 0 ℃ the reaction mixture was quenched with aqueous NaHCO3 (sat. sol.), then extracted with DCM. The organic layers were separated, dried (Na2SO4), filtered and the solvents evaporated in vacuoto yield intermediate 36 which was used as such in the next reaction step. Yield 1 g, quant.
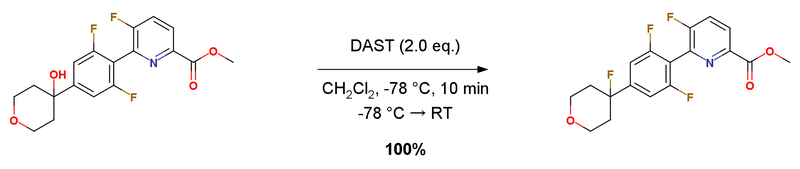
To a solution of methyl 6-(2,6-difluoro-4-(4-hydroxytetrahydro-2H-pyran-4-yl)phenyl)-5-fluoropicolinate (1.0 equiv.) in CH2Cl2 (0.04 M) at -78 ℃ under Ar was added methylDAST (2.0 equiv.). After addition, the solution was stirred under Ar at -78 ℃ for 10 minutes and then the bath was removed. The reaction was allowed to warm up to rt and quenched by addition of NaHCO3(sat.). The solution was diluted with EtOAc, washed with NaHCO3(sat.), NaCl(sat.), dried over MgSO4, filtered, concentrated, purified by ISCO SiO2 chromatography (0-100 EtOAc/n-heptanes) to yield methyl 6-(2,6-difluoro-4-(4-fluorotetrahydro-2H-pyran-4-yl)phenyl)-5-fluoropicolinate. 100% yield.
A solution of alcohol 51 (2.5 mg, 0.0052 mmol, 1.0 equiv) in CH2Cl2 (1.0 mL) at -78 ℃ was treated with DAST [(diethylamino)sulfur trifluoride] (1.3 μL, 0.010 mmol, ca. 2 equiv) and stirred at that temperature for 3 h. The reaction mixture was quenched by the addition of solid NaHCO3 (30 mg), warmed to 25 ℃, concentrated, and purified by flash chromatography (silica gel, EtOAc:CH2Cl2:MeOH, 10:10:1). The desired sarcodictyin analog 52 (2.5 mg, 99% yield).
Compound 2 (0.24 mmol) was dissolved in dry methylene chloride (3 mL) and to this solution, diethylaminosulfur trifluoride (DAST) (0.24 mmol) was added at -78 ℃ (dry ice/acetone) under nitrogen. The reaction was warmed to room temperature and stirred for 1 h. Progress of the reaction was monitored by TLC. The complete conversion of 2 into the desired product was achieved in 1 h. The reaction mixture was diluted with methylene chloride (10 mL), washed with brine and water, dried over Na2SO4, and concentrated to dryness under reduced pressure at 20 ℃ to obtain a crude reaction product. A silica gel flash column (n-hexane/ethyl acetate, 2:1, vol/vol. Yield: 98.5%, a colorless, viscous oily.
※本文より p 380
Compound 4 might have the inversion of an R- to S-configuration at C-12 to which the fluorine is bonded. Since stereochemistry was not studied, we preferred to show the bonding of fluorine at C-12 as a mixture of isomers (Scheme 1).
・近年、複数の研究者の貢献により、安定で取扱い容易なフッ素化剤が多数開発された。
(求核的フッ素化剤, 求電子的フッ素化剤の両分野において)
合成の自由度は格段に向上したが、何だかんだ言って、DAST も使い勝手が良い。
他のフッ素化剤と同様、DAST も一緒に準備しておくと便利。
・2級アルコールの場合は、一般的に立体反転を伴ってフッ素化が進行するが、
隣接基関与の影響等により、生成物の位置選択性、
立体選択性が影響を受ける場合もあるらしい。
(場合によっては、ちょっと気を付けた方が良いのかも)
計算化学に基づく研究例
→ DASTを用いたフッ化物アニオンによるSN2反応に関する理論的研究 (2000)
・フッ素原子の導入は、以前のエントリーでも述べた通り、
リガンドデザインにおいて極めて重要。
リガンドの代謝ブロック、塩基性の減弱(hERG リスクの改善)、
コンホメーションの操作等によく使われる。
↓応援クリックしてくれると励みになります!